Nickel-Cadmium Battery : Principle & Its Applications
The nickel-cadmium battery functions as a DC voltage source. Thanks to its attributes and benefits, it's displacing lead acid batteries and gaining traction lately. It boasts a small, sleek design, ideal for portability. Common applications include toys, calculators, and small DC motors. Essentially, it operates on the same principles as lead-acid batteries: a metal coated with cadmium, separated by layers, undergoes a redox reaction to generate DC voltage. With the aim of enhancing battery efficiency, an array of chemical elements are being employed, resulting in more streamlined constructions.
What Constitutes a Nickel-Cadmium Battery?
A nickel-cadmium battery is a device engineered to generate DC voltage through chemical reactions between its constituents. In this type of battery, a redox material serves as the foundation, surrounded by layers of nickel and a separator. Typically, the voltage of a nickel-cadmium cell hovers around 1.2 V. For practical purposes, 3 to 4 cells are usually combined in series to yield outputs ranging from 3.6 to 4.8 V.
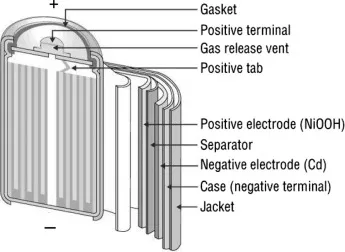
Unveiling the Theory Behind Nickel-Cadmium Batteries
The fundamental principle governing the operation of a nickel-cadmium battery aligns with that of other batteries. To enhance efficacy, nickel and cadmium are incorporated. A battery serves as a source of DC voltage, necessitating the presence of two potential points: positive and negative, or anode and cathode. In a nickel-cadmium battery, an initial layer of nickel oxide (NiO2) is enveloped around the redox, functioning as the cathode. Positioned atop the nickel oxide layer lies a separator layer saturated with KOH solution, crucial for facilitating the necessary OH negative ions during chemical reactions. Adjacent to the separator layer resides cadmium, fulfilling the role of the anode in the nickel-cadmium battery configuration. Refer to the accompanying diagram for a visual representation.
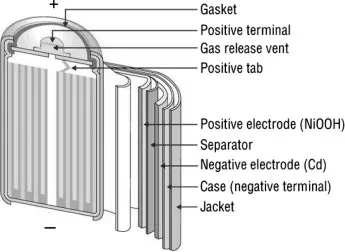
As illustrated in the schematic, nickel functions as the collector for the positive electrode, while the cadmium layer serves as the collector for the negative electrode. Acting as a barrier between these layers is the separator composed of either KOH or NaOH, which serves to furnish OH ions essential for the battery's operation. Additionally, the battery includes safety features such as a valve, sealing plate, insulation ring, insulation gasket, and an outer casing.
The insulation ring serves the purpose of isolating the two electrode layers, with the insulation gasket positioned nearby to accommodate the insulation ring and connect to the separator layer. Meanwhile, the outer casing shields the internal components from external hazards such as damage or mishandling.
It's crucial to acknowledge the inherent hazards associated with working with batteries due to the ongoing chemical reactions within them. Consequently, the battery casing should never be opened, as exposure to the internal layers could pose risks to the user. Similarly, when the battery is not in use, it's advisable to remove it from the device as a safety precaution.
Nickel Cadmium Battery Reactions
The chemical reactions characterizing the operation of a nickel-cadmium battery can be delineated as follows:
![]()
The initial equation depicts the interaction between the nickel cathode layer and the separator, yielding nickel oxide and OH ions. As previously elucidated, the separator layer serves the vital function of supplying the requisite OH ions for the reaction. To ensure the availability of H2O, the separator layer undergoes hydration initially, subsequently yielding H2O as a byproduct.
Conversely, at the anode, the cadmium layer likewise reacts with OH ions sourced from the separator layer, resulting in the formation of cadmium oxide and electrons. Notably, the electrons featured in both equations nullify each other, as do the OH ions. The residual equation, as denoted by the third expression, entails the amalgamation of nickel and cadmium with water, culminating in the production of nickel oxide and cadmium oxide.
In the initial equation, the interaction occurs between the nickel cathode layer and the separator, yielding nickel oxide and OH ions as outputs. As previously discussed, the primary role of the separator layer is to furnish the OH ions necessary for the chemical reaction. To ensure the presence of H2O, the separator layer is initially saturated with water for the initiation of the reaction, with H2O subsequently emerging as a byproduct.
Moving to the anode, the cadmium layer undergoes a similar process, reacting with OH ions derived from the separator layer, resulting in the formation of cadmium oxide and electrons. It's worth noting that the electrons in both equations effectively neutralize each other, as do the OH ions. The remaining equation, as depicted in the third expression, entails the combination of nickel with cadmium and water, yielding nickel oxide and cadmium oxide as the end products.
Nickel-Cadmium Battery Temperature Limits
The operational temperature range for nickel batteries spans from 0 to 45 degrees Celsius during charging and -20 to 65 degrees Celsius during discharging. Operating outside this temperature range renders the battery inoperative, potentially leading to hazardous situations such as explosions.
Nickel-Cadmium Battery Hazards
Nickel-cadmium batteries pose significant toxicity risks to human health. Cadmium, a heavy metal present in these batteries, poses various health hazards, affecting bodily functions directly. The average cadmium concentration in the human body is approximately 1 microgram per liter, primarily impacting the digestive system. Similarly, nickel, another constituent of these batteries, is harmful to the human respiratory system.
Nickel-Cadmium Battery Voltage Specification
Typically, each cell of a nickel-cadmium battery provides approximately 1.2 V. To achieve desired voltage levels, cells are interconnected either in series or parallel. Furthermore, the specific energy of these batteries is approximately 50-60 Wh per Kg, which is moderately higher than nickel-iron batteries but lower compared to nickel-zinc and nickel-metal hydride batteries.
The specific power output of nickel-cadmium batteries is around 200 W per kg, slightly surpassing that of nickel-iron batteries but falling short compared to nickel-zinc and nickel-metal hydride batteries. For nickel-metal hydride batteries, this figure ranges from 170 to 1000, whereas for nickel-iron batteries, it's approximately 100.
Regarding energy efficiency, nickel-cadmium batteries exhibit an efficiency range of 70-75%, which is higher than that of nickel-iron batteries but lower compared to nickel-zinc and nickel-metal hydride batteries. For nickel-metal hydride batteries, the efficiency ranges from 70 to 80%, while for nickel-iron batteries, it's approximately 60-70%.
Composition of Nickel-Cadmium Battery
Structurally, the nickel-cadmium battery closely resembles lead acid-based batteries, comprising three essential layers. Initially, there's a nickel layer, followed by a separator layer, and finally, a cadmium layer. The nickel layer serves as the positive electrode collector, while the cadmium layer functions as the negative electrode collector.
The separator layer sandwiched between these two layers is typically composed of KOH or NaOH, serving the crucial role of supplying OH ions necessary for the battery's operation. Additionally, the battery incorporates several components for safety and functionality, including a safety valve, sealing plate, insulation ring, insulation gasket, and an outer casing.
The insulation ring is strategically positioned to provide insulation between the nickel and cadmium layers, with the insulation gasket serving as its housing. The separator layer is intricately connected to this ring to ensure proper functioning.
Meanwhile, the outer casing is designed to shield the internal layers from external factors such as physical damage and mishandling. It's essential to note the inherent hazards associated with working with the battery due to ongoing chemical reactions. The combined layers, along with the separator layer, facilitate the necessary chemical reactions, resulting in the establishment of the potential difference essential for the battery's operation.
Working of Nickel-Cadmium Battery
The functionality of the nickel-cadmium battery hinges on the chemical interactions occurring within its layers. Serving as a direct current (DC) voltage source, the battery comprises two terminals: the anode and the cathode. During battery assembly, the cadmium layer is initially positioned atop the redox, functioning as the cathode terminal. Cadmium, being a heavy material, boasts excellent conductivity properties.
Immediately above the cadmium layer, separator layers are placed. These separator layers play a pivotal role in supplying the necessary OH ions crucial for chemical reactions. Specifically, the OH ions facilitate the reaction between the nickel cathode layer and the separator, yielding nickel oxide and OH ions as outputs. The essential function of the separator layer is thus reiterated: providing the OH ions essential for the chemical reaction. To initiate the reaction, the separator layer is initially saturated with water, resulting in the production of H2O as a byproduct.
On the anode side, the cadmium layer similarly interacts with OH ions sourced from the separator layer, leading to the formation of cadmium oxide and electrons. It's important to note that the electrons involved in these reactions effectively nullify each other, as do the OH ions. The remaining equation involves the combination of nickel with cadmium and water, resulting in the generation of nickel oxide and cadmium oxide. Subsequent to these chemical reactions, the flow of electrons ensues, thereby establishing the potential difference across the two terminals.
Varieties of Nickel-Cadmium Batteries
Classification of nickel-cadmium batteries primarily revolves around their size and available voltage. Sizing options range from AAA, AA, A, Cs, C, D, to F, each accompanied by distinct output voltage specifications. These batteries may manifest as cylindrical pipes or rectangular boxes, featuring diverse outer casings.
Pros and Cons
Advantages:
Capable of delivering high current output
Tolerates overcharging without adverse effects
Endures approximately 500 charging cycles
Disadvantages:
Cadmium, a constituent material, poses environmental concerns
Exhibits less tolerance to temperature fluctuations compared to alternative battery types
Applications of Nickel-Cadmium Batteries
Nickel-cadmium batteries find extensive use across a spectrum of applications, including toys, small DC motors, calculators, fans, computers, and more.
Therefore, having examined the applications, operational principles, and intricacies of nickel-cadmium batteries, it's imperative to explore alternative materials that can be paired with nickel, considering the hazardous nature of cadmium.
Related Articles
Different Types of Battery Chargers: How Do They Differ?
Lithium cr1620 Battery:Features, Specification and Applications
LR44 Battery: Everything You Need to Know
CR2450 vs CR2032 Battery: What are the Differences?
Cr2 vs Cr123: Which Battery Is Right for You?
CR1220 Battery Equivalent: Specification, Application and Features










