Dry Cell Battery vs Wet Cell Battery: Differences
Introduction
A dry cell battery is a small and closed cell with the electrolyte in a paste form or solid state, thereby making it more convenient and safer to carry around. A wet cell battery, on the other hand, uses a liquid electrolyte that can hold a large amount of energy but needs maximum maintenance. Although the two types of power sources are similar in the roles they operate, they have been constructed differently to suit different power requirements and the environment. This paper will give a comparative analysis of dry cell and wet cell batteries in regard to their design, functionality and the best applications.
What is a Dry Cell Battery?
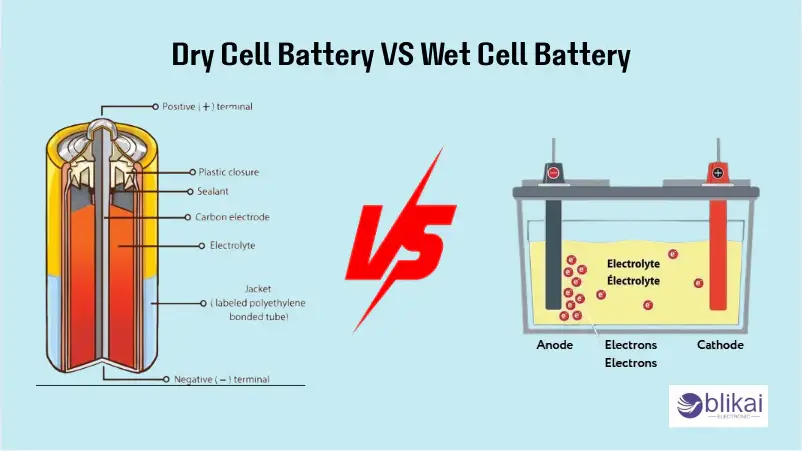
A dry cell battery is any electrochemical cell in which the electrolyte is in the form of a paste or gel, but not in the form of a liquid. This reduces the size, makes dry cell batteries leak-proof, and can be used in a portable manner. A container made of zinc (a container of the anode), a rod made of carbon (a container of the cathode), and an electrolyte paste facilitating the movement of ions are the most important elements in a dry cell. The reason behind this is that when these elements are combined, the battery can store and produce electrical energy when required.

How Dry Cell Batteries Function
The operation of a dry cell battery is founded on a chemical reaction between the anode (zinc) and the cathode (carbon) under the support of the electrolyte paste. When the battery is being used, the current of electrons empties from the anode into the external circuit to the cathode, and the process generates an electric current that drives some device. The electrochemical process that occurs at the anode and cathode generates energy, and the electrolyte is consumed to maintain the movement of ions between the electrodes. The design is capable of providing a consistent and predictable stream of power until the chemical elements are depleted.
Common Examples of Dry Cell Batteries
One of the most frequent types of dry cell batteries is the AA, AAA and 9V batteries that are used in a variety of consumer electronics. Such devices typically use AA and AAA batteries, such as remote controls, flashlights and even toys and 9V batteries are used in such devices as smoke detectors, hearing aids and even portable radios. The majority of such batteries are inexpensive, easily available and can be used in a myriad of different devices daily and hence a popular choice at home and in industry.
What is a Wet Cell Battery?
An electrochemical cell that uses a liquid electrolyte to help in facilitating the chemical reactions that will yield electrical energy is referred to as a wet cell battery. A wet cell battery is a battery that consists of the positive and the negative plates (typically of lead in lead-acid batteries) and a liquid electrolyte (typically sulfuric acid in a lead-acid battery), and a battery case to offer enclosure of the various components. The reason is that the electrolyte is needed as it helps the flow of the ions between the plates; therefore, at this point, it is referred to as such that the battery is able to store as well as release energy.
How Wet Cell Batteries Function
The electrolyte in a wet cell battery is a liquid solution (usually sulfuric acid and water solution in lead-acid batteries), through which ions move between the anode (negative plate) and the cathode (positive plate). During active use of the battery, the sulfuric acid decomposes into sulfuric and lead plates, which form a chemical reaction resulting in electrical current. This current flows through the external circuit to power devices. The liquid state of the electrolyte enables it to have a better energy capacity than dry cell batteries. Nevertheless, wet cell batteries are larger and more difficult to maintain in order to achieve the best performance due to the liquid electrolyte.
Common Applications of Wet Cell Batteries
Wet cell batteries are mostly applicable in those applications that demand a good level of power and energy storage. The most significant use has been in automobiles where lead-acid batteries are employed to have the cars, trucks, and motorcycles starting, lighting and ignition (SLI) systems. A wet cell battery is also widely used in deep-cycle batteries, which are applied in the solar energy system, marine boats and recreation vehicle (RV) applications. These batteries can supply a steady amount of power in the long term, and they are best suitable in scenarios where power needs are more or when the power backup is required.
Comparison Table of Dry Cell Batteries and Wet Cell Batteries
Key Differences Between Dry Cell and Wet Cell Batteries
Electrolyte Type
The initial distinction between the dry cell and wet cell batteries is in the type of electrolyte used. The electrolyte in dry cell batteries is solid or paste-like, and is typically a mixture of ammonium chloride or zinc chloride. This hard-state allows the battery to be compact and leak-free, and therefore applicable in handheld devices. Wet cell batteries, however, make use of some liquid electrolyte, as in the lead-acid battery, usually a dilution of sulfuric acid in water. Liquid electrolyte provides a more and more constant ionic flow between the electrodes, providing greater energy capacity. However, the wet cell type of battery is the most sensitive because the liquid state of the electrolyte could leak or evaporate over time, hence its performance.
Design and Portability
Dry cell batteries are obviously superior when it comes to design and portability. Dry cell batteries are small, closed-ended and, hence, lightweight, easy to carry and highly portable. They are widely found in the consumer electronics used by everyday people, including flashlights, remote controls and toys where size and convenience are important. Wet cell batteries, however, are normally much larger and heavier in weight because they require a liquid electrolyte and more massive internal parts. This has led to wet cell batteries being less portable and being larger in size, therefore more suitable for fixed applications, including automobiles and backup power systems. Also, wet cell batteries need more regular servicing, including monitoring the electrolyte levels and removing terminals that are out of order in order to make them work well, which may complicate their usage in the case of users who want to have hassle-free solutions.
Energy Output and Lifespan
Compared to dry cells, wet cell batteries tend to be more efficient in energy production and have extended life, particularly when continuous power is needed. Wet cell batteries are developed to support more power load and generally have greater energy production, hence are best suited to high loads, like in a car or an industrial machine. They also have a higher ability to recharge and recycle more than once, thus having a better lifespan than dry cell batteries. Dry cell batteries are not ideal in the construction of lower power devices, as they are not as capable of storing energy and their operation duration is limited, and they tend to need replacement as soon as the power is drained. The wet cells, especially the lead-acid batteries, have a life of several years when maintained properly, whereas the dry cells last months. Wet cell batteries are therefore preferable in long-term high-demand applications, even though the initial cost and maintenance are higher.
Cost
Dry cell batteries do not cost much in terms of purchase cost as well as maintenance cost. They are an inexpensive way to go when it comes to the numerous consumer products due to their simplicity and easy-to-use nature. Wet cell batteries, on the other hand, are more expensive to start with because they are larger, more complex, and also the electrolytes are in liquid form. Furthermore, a wet cell battery usually requires constant care, such as ensuring that the electrolyte level in the batteries is at the correct level and that the terminals are clean, and this makes the batteries more expensive to own. Wet cell batteries can be a better value even at these increased costs when a certain degree of power is required to be high, long life, and wide power capability are desired, e.g., in an automobile or industrial environment. Hence, although dry cells are cheaper in daily, low-power operating conditions, wet cells are an economical option in high-power and long-term operations.
Which is Better: Wet Cell or Dry Cell Battery?
The two have distinct advantages, but one can be better than the other based on availability or power requirements, maintenance readiness, portability, and price.
For Everyday Consumer Electronics
Batteries based on dry cells are the obvious choice when it comes to low-power devices such as remote controls, toys, flashlights, among others. They are small, portable and cheap, making them the most convenient among the consumers. Dry cells do not need any maintenance and can be used straight off the box, and it is therefore a perfect match with the devices that need small bursts of power on a short-term basis.
For High-Power, Long-Term Applications
Wet cell batteries tend to be better when there is a high power requirement, which involves a long flow of energy, like an automobile, storage of solar energy and a source of power during a power outage. They are perfect in industries and machinery with high regard to reliability and time in performance because they can store more energy, carry heavy loads and can be recharged numerous times. However, wet cells involve regular maintenance, and this may not be a convenient thing for all users.
Conclusion
There are also pros and cons to dry and wet cell batteries, and they can be deployed to be used in different applications depending on the power requirement, upkeep, and environmental factors. The energy capacity, the lifespan, the maintenance requirements, and the cost are some of the factors that users need to consider when choosing a type of battery that is the most suitable to use. With technology still developing, both types of batteries may continue to be separated further with the introduction of additional inventions in battery chemistry and design to create new and specific applications for different energy needs.
Some images are sourced online. Please contact us for removal if any copyright concerns arise.
SR927W Battery Guide: Specs, Equivalents, & Replacement
18350 Battery: Size, Applications, & Comparisons
E90 Battery Guide: Specs, Applications& Equivalents
51R Battery: Size, Compatibility, & Applications
CR2430 Battery: Features, Lifespan & Replacement Guide
14500 Battery Explained: Features, Comparison & Lifespan
A23 Battery: Applications, Equivalents, Lifespan, & Key Differences
The Complete U1 Battery Guide: Types, Variants, & Applications
4D Battery Guide: Size, Types & How to Extend Its Life
2CR5 Battery: Specs, Equivalents & Applications










